Blockchain - Transforming the Life Sciences industry
06/26/2020
COVID-19 has revealed systemic weakness in a wide variety of industries and processes, and the supply chain for medical supplies is a perfect example.
Regional lockdowns amplify the shortages by disrupting supply chains, with upstream supplier closures resulting in a cascading effect leading to downstream manufacturer disruptions.
With extremely limited visibility into the processes behind the supply chain, manufacturers are unable to anticipate upstream shortages in time to develop alternatives. Meanwhile, consumers, in their scramble to secure essential medical supplies, become particularly exposed to the risks of counterfeits and substandard goods in a fit of panic buying when demand exceeds supply.
While medical supply chains and medicines development may not at first seem connected, the weaknesses in both arenas are largely symptoms of the same issue: inadequate data management. In the digital age, data is the most sought after commodity, but legacy systems have prevented us from exploiting the resource to anywhere near its full potential.
Blockchain-supported decentralized systems provide a better way to manage data:
- by overcoming the challenges related to security, privacy, protection of IP, and adherence to regulations
- these systems offer the potential to unleash the power of data— to the benefit of everyone involved
For example:
- Developing supply chains with a universal view, for manufacturers to anticipate downstream shortages in time to develop alternatives
- Improved track and trace systems that give consumers more confidence in the medical products they buy
- Patient data can be securely across medical ecosystems, resulting in better patient care while providing additional research opportunities
- Pharmaceutical companies can share previously siloed data empowering them to leverage AI and improve coordination across stakeholders in the development of new medicines
The case for Blockchain in Life Sciences
Blockchain is becoming increasingly relevant to address the perennial challenges of trust and speed in the life sciences supply chain. For example, enabling data driven business decisions in forecasting the flow of goods and having visibility into the origin of materials and inputs has always been difficult, partly due to the technologies involved and partly because suppliers are mistrustful about sharing data they fear will be abused by downstream customers, impacting their ability to price products effectively.
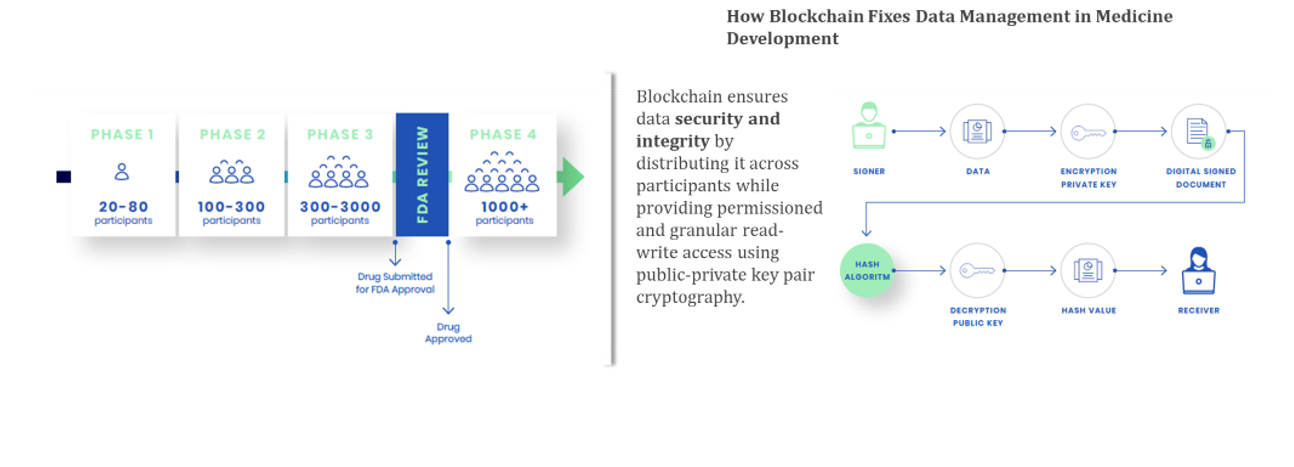
Data Management is the Key
Medicines development makes use of the scientific method whereby hypotheses are developed then tested. Data is of course central to both of those steps. An abundance of data will lead to high quality hypotheses, while even more data are needed to prove the hypotheses. The ability to gather and share data then, is central to the medicines development process.
To understand how this works at a practical level, let’s look at clinical trials, the backbone of medicines development. Before any medicine can be approved, it must go through a series of clinical trials. These are an attempt to standardize the application of the scientific method to medicines development.
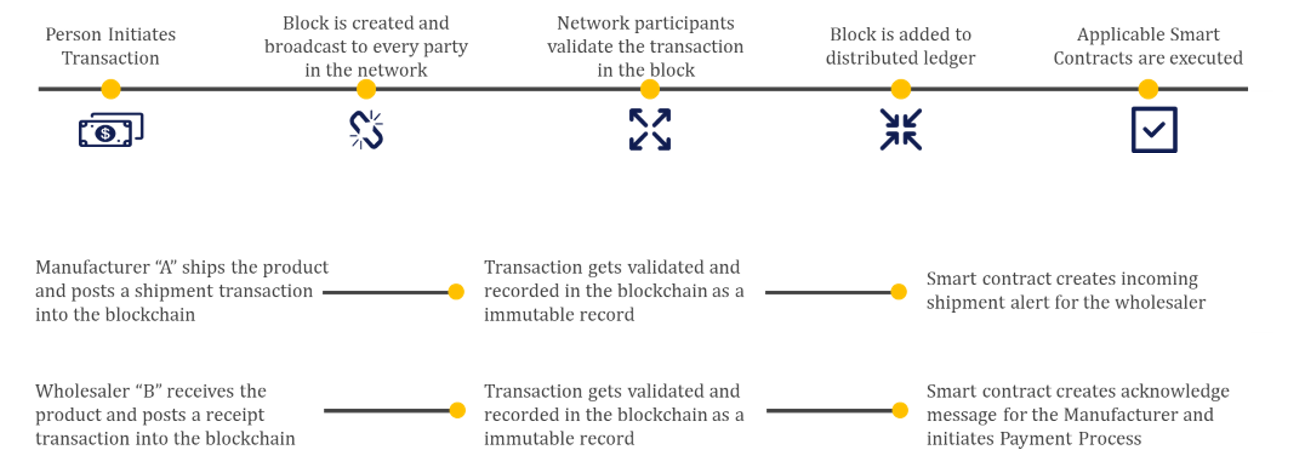
Use Cases:
1. Provenance
One of the most complex life sciences supply chain challenges has been the ability to effectively track the origin of a product (or therapy) from raw materials to the finished product. Despite the various efforts of full chain of custody systems that exist today, the fragmentation of systems between trading partners opens the risk for fraud. Blockchain technology is an ideal solution, given that no single organization is responsible for provenance.
Organizations across the life sciences ecosystem benefit from having authentic products in the supply chain, ensuring brand integrity and improved patient outcomes by delivering authentic products to the patient. Blockchain enables the idea of a “digital passport” for a product, containing all relevant information for each component or ingredient, including instructions and patient adherence information from the packaging.
2. Serialization and Track & Trace
For the last two decades, regulators across the globe have been implementing requirements for unique product identification to deliver greater security of finished products through the supply chain. These requirements have the aspiration to eliminate counterfeit and diverted products, ultimately contributing to increased patient safety.
Like provenance, one of the major challenges with track and trace is the effective exchange of data across the ecosystem of partners - from the pharmaceutical manufacturers, to wholesale distributors, to dispensers.
With the use of blockchain, supply chain partners can more effectively and securely share data across the supply chain and, eventually, with the end patient.
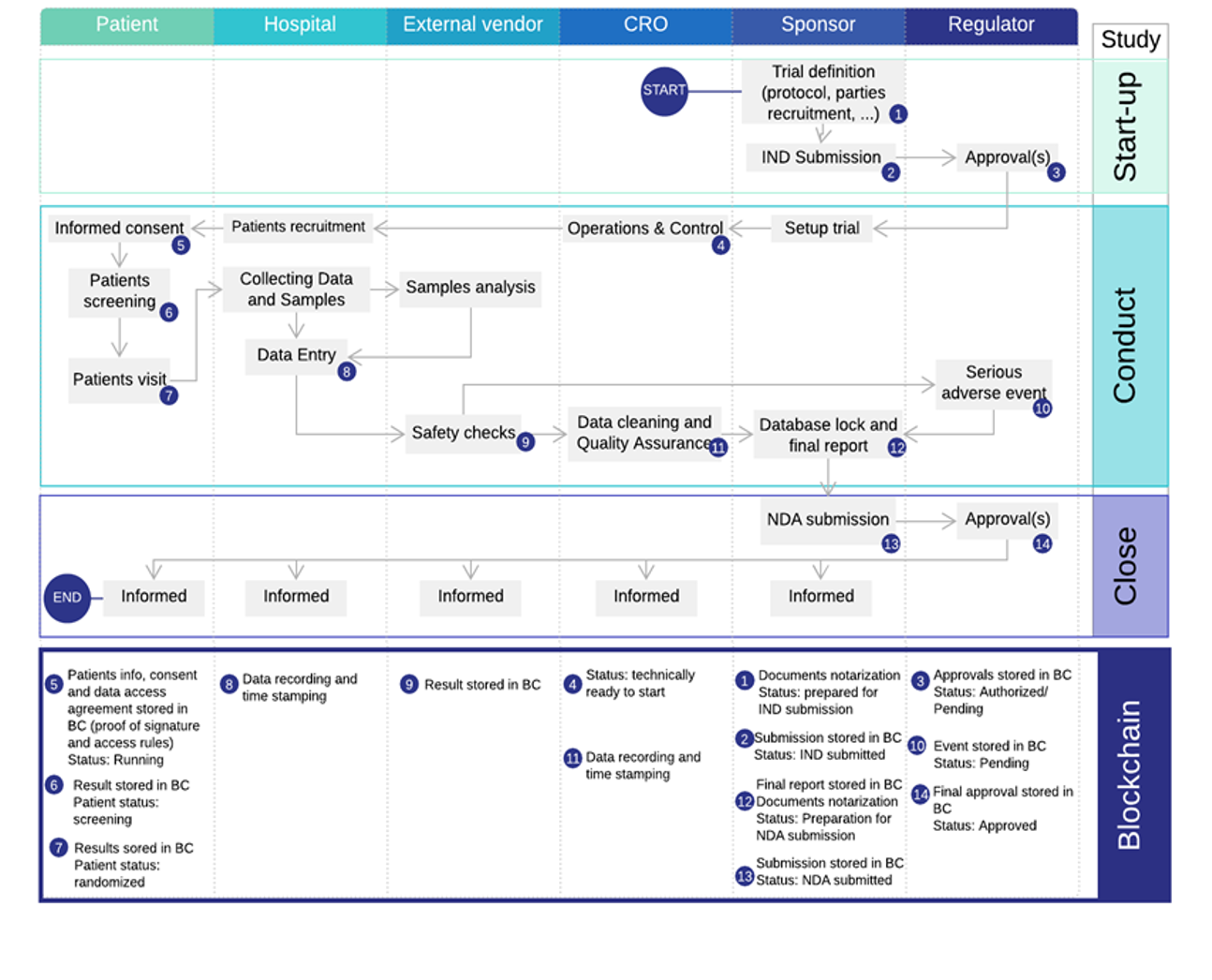
3. Clinical Trials
Industry Challenges:
- Data in Silos
- Complex data sharing
- Low patient involvement
- Lack of transparency
- Long trial lead times
Solutions benefits:
- Full transparency & real-time data sharing
- Better patient care & higher patient involvement
- Accelerated medical innovation
With the use of blockchain, supply chain partners can more effectively and securely share data across the supply chain and, eventually, with the end patient.
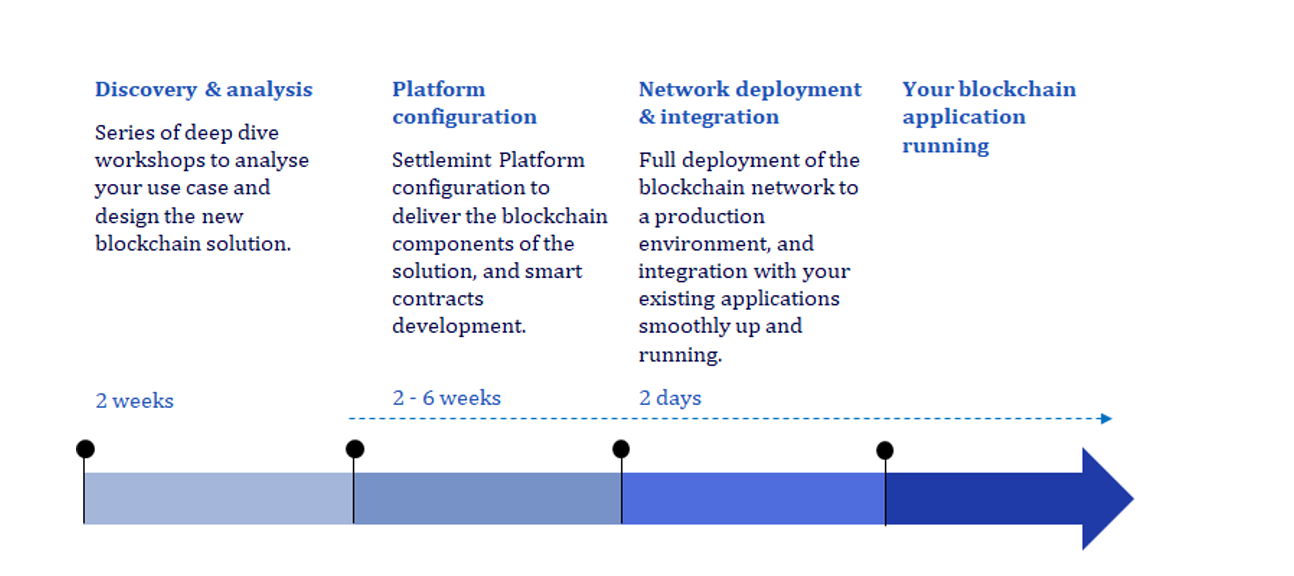
Starting the Blockchain Journey
Within the next few years, approximately 30% of life sciences companies plan to utilize blockchain, opening new business opportunities and addressing challenges of the past.
Supply chain applications of blockchain are endless for life sciences companies given the requirements for specialized medicines and therapies. Those that start now—who are willing to experiment, fail fast and innovate based on that experience—will be the organizations to achieve competitive advantage and improve patient outcomes.
About NTT Data: NTT DATA has a strategic Partnership with SettleMint, The # 1 Lowcode platform for Enterprise Blockchain Applications. To find out more please visit us at www.nttdata.com/sg/en
If you have any queries, please reach out to our Business Lead for Intelligent Automation: patankar.pramod@nttdata.com
